Spravato
Spravato services offered in Arizona Ave and Ray Road, Chandler, AZ
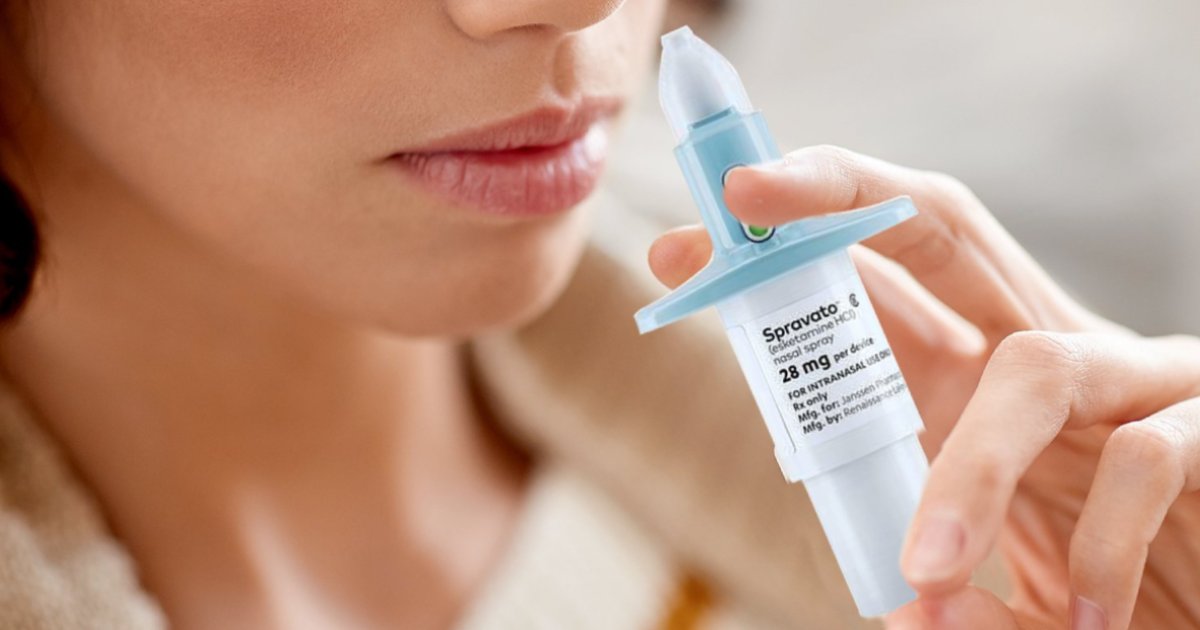
Spravato is intranasal spray for treatment resistant depression. If you struggle with treatment- resistant depression, there’s a different choice to turn to.
Spravato, an intranasal spray, represents a significant advancement in the treatment of treatment-resistant depression. This medication offers hope to individuals who have struggled to find relief with traditional antidepressant therapies. By targeting specific receptors in the brain, Spravato works to alleviate symptoms of depression and improve overall mental well-being. Its innovative delivery method allows for rapid absorption, offering a more efficient and effective treatment option for those in need. With Spravato, individuals experiencing treatment-resistant depression have a new avenue for finding relief and reclaiming their lives from the grip of this debilitating condition.
Spravato itself is not associated with specific symptoms. However, common side effects that may occur after administration of Spravato include dizziness, dissociation, sedation, and increased blood pressure. It’s essential to consult a healthcare professional for a comprehensive understanding of potential side effects and how to manage them effectively.
The use of Spravato (esketamine) may pose certain risks, and it’s important to consider various factors before starting treatment. Some potential risk factors associated with Spravato include:
History of substance abuse: Individuals with a history of substance abuse may be at increased risk of misuse or dependence on Spravato due to its potential for dissociative effects.
Cardiovascular conditions: Spravato may increase blood pressure and heart rate shortly after administration, which could pose risks for individuals with pre-existing cardiovascular conditions.
Respiratory conditions: As a nasal spray, Spravato may cause irritation or adverse reactions in individuals with respiratory conditions such as asthma or chronic obstructive pulmonary disease (COPD).
Psychiatric disorders: People with certain psychiatric disorders, such as psychosis or bipolar disorder, may experience exacerbation of symptoms or adverse reactions when using Spravato.
Liver impairment: Spravato is metabolized in the liver, so individuals with liver impairment may experience altered metabolism and potential toxicity.
Pregnancy and breastfeeding: The safety of Spravato use during pregnancy and breastfeeding has not been fully established, so it’s important for pregnant or breastfeeding individuals to discuss the risks and benefits with their healthcare provider.
Spravato (esketamine) is administered as a nasal spray and is typically used in combination with an oral antidepressant medication. The treatment process for Spravato typically involves the following steps:
Screening and evaluation: Before starting Spravato treatment, individuals undergo a comprehensive evaluation by a qualified healthcare provider to assess their suitability for the medication. This evaluation may include a review of medical history, a physical examination, and a mental health assessment.
Prescription and dosing: If deemed appropriate, a healthcare provider will prescribe Spravato along with an oral antidepressant medication. The initial dosing and frequency of Spravato administration are determined based on the individual’s specific needs and response to treatment.
Administration: Spravato is self-administered as a nasal spray under the supervision of a healthcare provider in a certified treatment center. Individuals typically receive the medication in the healthcare setting and are monitored for a period of time after administration to monitor for any adverse reactions.
Ongoing monitoring and adjustments: Throughout the course of treatment, individuals are closely monitored by their healthcare provider to assess their response to Spravato and any potential side effects. Dosage adjustments or changes to the treatment plan may be made based on the individual’s progress and tolerability.
Maintenance therapy: For many individuals, Spravato treatment is part of a broader maintenance therapy plan aimed at managing symptoms of depression over the long term. This may involve continued use of Spravato along with other medications and therapeutic interventions.
It’s important for individuals undergoing Spravato treatment to actively participate in their treatment plan, attend regular follow-up appointments, and communicate openly with their healthcare provider about any concerns or changes in symptoms. Additionally, adherence to the prescribed dosing schedule and safety guidelines is essential for optimizing the effectiveness and safety of Spravato treatment.